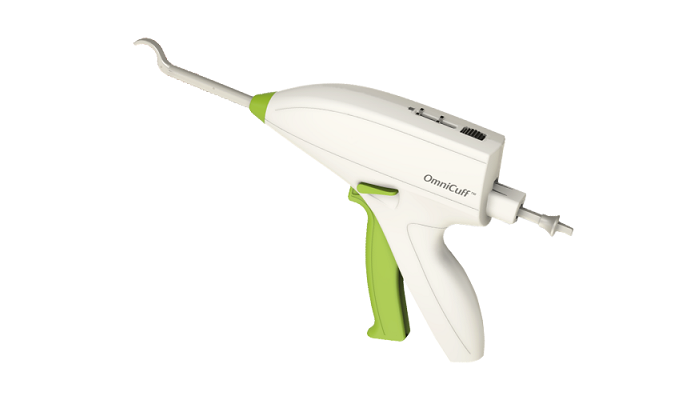
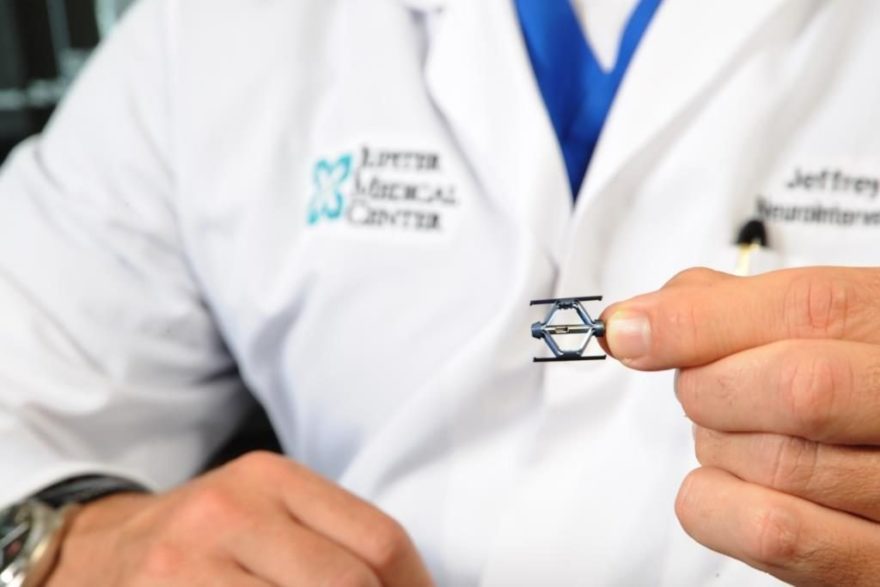
Magal, Israel, December 7, 2018 – MinInvasive Ltd., developer of the OmniCuff™ device for treatment of rotator cuff repair, reported positive progress following its initial two months of limited market release activity in the US with 26 OmniCuff™ System cases successfully concluded to date.
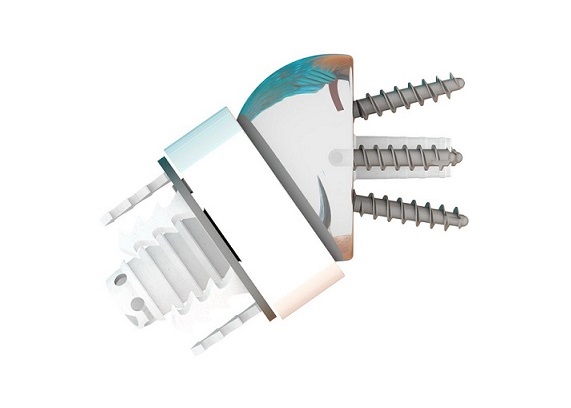
Shoulder rotator cuff repair is a fast-growing market segment within the sports medicine market, with over one million procedures performed annually worldwide. The OmniCuff™ System is an arthroscopic transosseous rotator cuff repair device that eliminates the need for suture anchors. The device provides the advantages of minimally invasive transosseous repair in a disposable device. MinInvasive CEO Ronen Raz stated, “We are very pleased that within the first 8 weeks of limited US market release, the OmniCuff™ System has been successfully used by several very reputable sports medicine surgeons in 26 rotator cuff repair procedures”.
Joseph Abboud, MD, Professor of Orthopedic Surgery at the Rothman Institute at Thomas Jefferson University in Philadelphia, recently performed several OmniCuff cases and confirmed that he is “extremely pleased with the technical ease and reproducibility of the device, as well as the tremendous potential for direct cost savings.”
Walter Stanwood, an Orthopedic surgeon at Beth Israel Deaconess Hospital in Plymouth, Massachusetts has completed over a dozen surgeries with the Omnicuff, and commented that the System offers “a very straightforward technique that minimizes steps, with an elegant device to consistently create transosseous fixation in a rotator cuff repair model. OmniCuff has enormous potential in any location that a tendon-bone repair is performed.”
Nikhil Verma, MD Professor and Director of the Division of Sports Medicine and Shoulder Surgery at Rush University Medical Center in Chicago, remarked that the device “allows for a seamless transition between traditional anchor-based repair, and an anchorless solution. In addition, the availability of a technologically advanced and easy to use anchorless device has the potential to disrupt the market with a lower cost solution which is of paramount importance given recent movement of these types of cases to outpatient facilities and the emphasis on value-based care.”
Frank Cordasco, MD, MS, Professor of Orthopaedic Surgery at the Hospital for Special Surgery and the 35th President of the American Shoulder and Elbow Surgeons has observed “remarkably diminished pain in the post-operative period in patients treated with the OmniCuff System compared to those treated with the transosseous equivalent rotator cuff repair technique during the first 3 weeks following surgery. This has resulted in a decreased need for Opioid medications in the patients treated with the OmniCuff System”.
MinInvasive plans to gradually expand the availability of the device in the US and is currently in the process of securing CFDA approval for OmniCuff™ in China, another huge potential market for innovative medical technologies.
MinInvasive Ltd.
MinInvasive has developed the OmniCuff™ System – an innovative device enabling arthroscopic, transosseous rotator cuff repair. In 2016, MinInvasive entered into a strategic partnership with MicroPort Scientific Corporation to obtain CFDA approval for the OmniCuff™ system. MinInvasive has validated manufacturing of its new OmniCuff™ disposable version and initiated a limited market release in early October in the US.
Contact:
Ronen Raz, CEO, MinInvasive Ltd. 137 Hashachaf, Magal, Israel
ronen.raz@mininvasive.com
+972-54-6222410