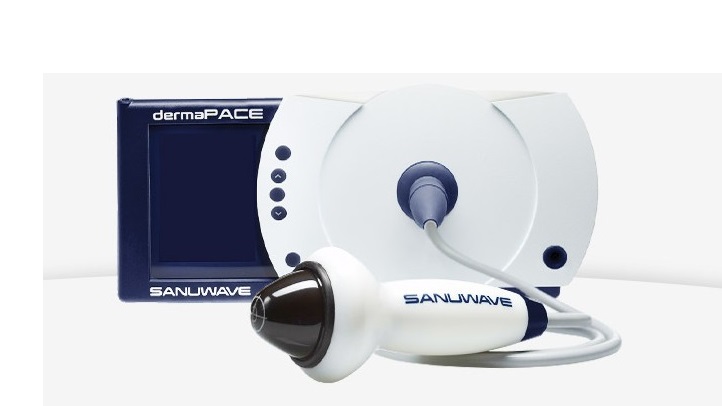
SUWANEE, GA, March 27, 2018 (GLOBE NEWSWIRE) — SANUWAVE Health, Inc. (OTCQB: SNWV) is pleased to announce that during its participation at the European Wound Management Association (EWMA) Conference in Krakow, Poland on May 9 – 11, 2018, the Company will present new pre-clinical and clinical research associated with the dermaPACE® System, SANUWAVE’s flagship medical care device. The dermaPACE System is currently CE Marked within Europe for the treatment of various acute and chronic conditions of the skin. In December 2017, SANUWAVE received US FDA clearance for the treatment of Diabetic Foot Ulcers (DFUs), which makes the dermaPACE System the only approved focused shockwave system in the US market for such indication. SANUWAVE will exhibit at expo booth D508 in Dunaj Hall together with Matek and Ortho Medico, the Company’s Italian and BeNeLux distribution partners.
For the 2018 EWMA conference the following four (4) abstracts, sponsored by SANUWAVE, were accepted that support the use of the dermaPACE System for various wound management conditions:
– “Pulsed Acoustic Cellular Expression as a Protective Therapy against Ischemia-Reperfusion Injury in a Cremaster Muscle Flap Model”, by Siemionow M., Krokowicz L., Klimczak A., Cwykiel J., Mielniczuk M. – this animal study tested PACE® conditioning mechanism of action on microcirculatory hemodynamics in ischemia-reperfusion injury model. The results showed that pre- and post-ischemic PACE conditioning improved functional capillary density and stimulated angiogenesis as confirmed by up-regulated VEGF expression. Furthermore, post-ischemic PACE conditioning correlated with decreased expression of early proinflammatory factors (iNOS, CCL2, CXCL5). Both types of PACE conditioning ameliorated deleterious effect of ischemia-reperfusion injury on microcirculatory hemodynamics of muscle flaps. The importance of this study is that it lays the basic foundation for using PACE technology for improving outcomes after grafting. The presenter of the study will be Dr. Maria Siemionow, a Polish transplant surgeon and scientist, who performed the world’s first near-total facial transplant successfully at Cleveland Clinic, U.S.A. Dr. Siemionow was twice honored with the James Barrett Brown Award for best publication in a plastic surgery journal in 2004 and 2007 and received the Folkert Belzeer Award in 2001. She is the recipient of the Commander’s Cross Polonia Restituta award given by the President of Poland in 2009, and in 2014, she received the Great Immigrants Award from the Carnegie Foundation of New York. Dr. Siemionow is now affiliated with University of Illinois at Chicago College of Medicine, as a professor of orthopedic surgery and Director of Microsurgery Research.
– “Healing Venous Leg Ulcers with Pulsed Acoustic Cellular Expression (PACE) Therapy” by Miller C., Kapp S., Green J., McGuiness W., Woodward M. – this is a case series for venous leg ulcers (VLUs) with six (6) participants with a mean age of 78.67 (SD=9.97) and wound duration of 34.80 weeks (SD=23.02) who were treated with the dermaPACE System. Positive wound healing trends were observed for all patients. A reduction in wound size was observed for five patients and although one patient developed an increased wound size, the tissue quality was improved. The treatment was easy to implement for clinicians and client acceptability was very good; some pain was recorded during 2 treatments (of 17 treatments) and occurred when the patient was reporting pain at the dressing change in general. The importance of this study is that it shows that the dermaPACE System has promising effects when used to treat venous leg ulcers. The presenter at this study will be Dr. William McGuiness, who is an Associate Professor at La Trobe University, Victoria, Australia and the Director of the La Trobe Alfred Clinical School of Nursing and Midwifery. Dr. McGuiness has been involved in wound management since 2000, as both an educator and a clinician. He was the President of the Australian Wound Management Association (AWMA).
– “The New Life” of a Stuck Wound: The Real ESWT Mission”, by Cassino R. – in this study the dermaPACE System was used for twelve (12) patients with chronic skin lesions of known etiology (pressure ulcers, venous leg ulcers, diabetic foot lesions), older than 6 months, not responding to ongoing treatments for at least 4 weeks or in a worsening status. The dermaPACE System treatment sessions were performed once a week for 4 weeks. The follow up was performed after the 4 applications, and then again after 5 and 10 weeks from the first assessment, by evaluating the Healing Rate (HR) and Wound Area Reduction (WAR). All wounds improved after the PACE treatment with a mean HR of 0.139 cm/week (ratio of A/P, where A = area in square centimeters and P = perimeter in centimeters) and a mean WAR/week of 28.6%. This trend of improvement continued for 10 other weeks. Also, at the end of the follow-up the mean total WAR was about 74%. The presenter at this study will be Dr. Roberto Cassino, who is affiliated with the Interdepartmental Center Team of Diabetic Foot from Istituto Clinico Città Studi – Milano, Italy. Dr. Cassino is an expert in geriatrics, gerontology, and general surgery.
– “Extracorporeal Shock Wave Therapy Improves Healing of Chronic Ulcers and Patients’ Quality of Life”, by Leemans G., Gutermuth J., Janmohamed S. – this study used ten (10) patients with chronic, therapy-resistant ulcers. The results demonstrated improvement in 10 out of 10 investigated ulcers in all measured parameters after Extracorporeal Shock Wave Therapy (ESWT). A mean decrease in wound surface and wound depth of 71% and 60% respectively, were observed six weeks after the first treatment session. Chronic ulcers and pain had a significant impact on patients’ Quality of Life (QoL), and to assess QoL, patients were asked to complete a Dermatology Life Quality Index (DLQI) questionnaire and pain was evaluated with a Visual Analogue Scale (VAS). A mean decrease in DLQI scores (i.e. an increase in QoL) of 60% was observed and a significant mean decrease of 62% in VAS scores (i.e. a decrease in the amount of pain) after ESWT could be seen. In comparison with other additional treatment options such as negative-pressure wound therapy and hyperbaric oxygen, ESWT is a non-time-consuming method and does not have systemic side effects. The importance of this study is that it shows that the dermaPACE System has significant success in treating chronic ulcers that were very difficult to heal using other technologies. The presenter of this study will be Dr. Gaëlle Leemans, who has medical studies at Vrije Universiteit Brussel (Free University Brussel) with the specialization in wound healing and dermatopathology. Dr Leemans is now affiliated with the Department of Dermatology, from the Universitair Ziekenhuis Brussel (University Hospital Brussels), Brussels, Belgium.
Dr. Maria Siemionow, Dr. William McGuiness, Dr. Roberto Cassino, and Dr. Gaëlle Leemans will be available to answer questions at SANUWAVE’s booth on May 10th, between 2 and 4 pm. Please contact Dr. Iulian Cioanta via e-mail at iulian.cioanta@sanuwave.com; in case that you want to meet individually with any the authors of the above studies.
“We are excited to exhibit at EWMA in Krakow, Poland, and to have our distinguished guests presenting scientific studies that show additional evidence regarding our PACE technology effectiveness in treating various types of hard-to-heal wounds and also its mechanism of action. Our FDA clearance for the dermaPACE System is built on solid clinical scientific evidence provided by two US-based randomized-controlled clinical trials enrolling 336 subjects, which showed that SANUWAVE’s technology is safe and effective in the treatment of Diabetic Foot Ulcers. Within a few weeks of initial treatment, wounds treated with the dermaPACE System showed a reduction in area at superior rates compared to control subjects. The support and interest coming from the wound care community has been overwhelmingly positive, both domestically and internationally. The scientific presentations for the EWMA 2018 conference validate our continuous commitment and strong belief in clinically based research in order to find new avenues for our technology and provide improved treatment options for patients, healthcare professionals, and clinicians,” stated Kevin A. Richardson, CEO and Chairman of the Board.
If you are interested in distribution opportunities for SANUWAVE in Europe or in scheduling a meeting with the SANUWAVE team during the conference, please contact Dr. Iulian Cioanta via e-mail at iulian.cioanta@sanuwave.com; otherwise, please stop by our booth D508 in Dunaj Hall at the conference in May.
About SANUWAVE Health, Inc.
SANUWAVE Health, Inc. (www.sanuwave.com) is a shock wave technology company initially focused on the development and commercialization of patented noninvasive, biological response activating devices for the repair and regeneration of skin, musculoskeletal tissue and vascular structures. SANUWAVE’s portfolio of regenerative medicine products and product candidates activate biologic signaling and angiogenic responses, producing new vascularization and microcirculatory improvement, which helps restore the body’s normal healing processes and regeneration. SANUWAVE applies its patented PACE technology in wound healing, orthopedic/spine, plastic/cosmetic and cardiac conditions. Its lead product candidate for the global wound care market, dermaPACE, is US FDA cleared for the treatment of Diabetic Foot Ulcers. The device is also CE Marked throughout Europe and has device license approval for the treatment of the skin and subcutaneous soft tissue in Canada, South Korea, Australia and New Zealand. SANUWAVE researches, designs, manufactures, markets and services its products worldwide, and believes it has demonstrated that its technology is safe and effective in stimulating healing in chronic conditions of the foot (plantar fasciitis) and the elbow (lateral epicondylitis) through its U.S. Class III PMA approved OssaTron® device, as well as stimulating bone and chronic tendonitis regeneration in the musculoskeletal environment through the utilization of its OssaTron, Evotron® and orthoPACE® devices in Europe, Asia and Asia/Pacific. In addition, there are license/partnership opportunities for SANUWAVE’s shock wave technology for non-medical uses, including energy, water, food and industrial markets.
Forward-Looking Statements
This press release may contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, such as statements relating to financial results and plans for future business development activities, and are thus prospective. Forward-looking statements include all statements that are not statements of historical fact regarding intent, belief or current expectations of the Company, its directors or its officers. Investors are cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties, many of which are beyond the Company’s ability to control. Actual results may differ materially from those projected in the forward-looking statements. Among the key risks, assumptions and factors that may affect operating results, performance and financial condition are risks associated with the regulatory approval and marketing of the Company’s product candidates and products, unproven pre-clinical and clinical development activities, regulatory oversight, the Company’s ability to manage its capital resource issues, competition, and the other factors discussed in detail in the Company’s periodic filings with the Securities and Exchange Commission. The Company undertakes no obligation to update any forward-looking statement.
For additional information about the Company, visit www.sanuwave.com.
Contact:
Millennium Park Capital LLC
Christopher Wynne
312-724-7845
cwynne@mparkcm.com
SANUWAVE Health, Inc.
Iulian Cioanta, Ph.D.
Vice President of Research and Development
678-578-0106 (Office)
iulian.cioanta@sanuwave.com