March 05, 2018
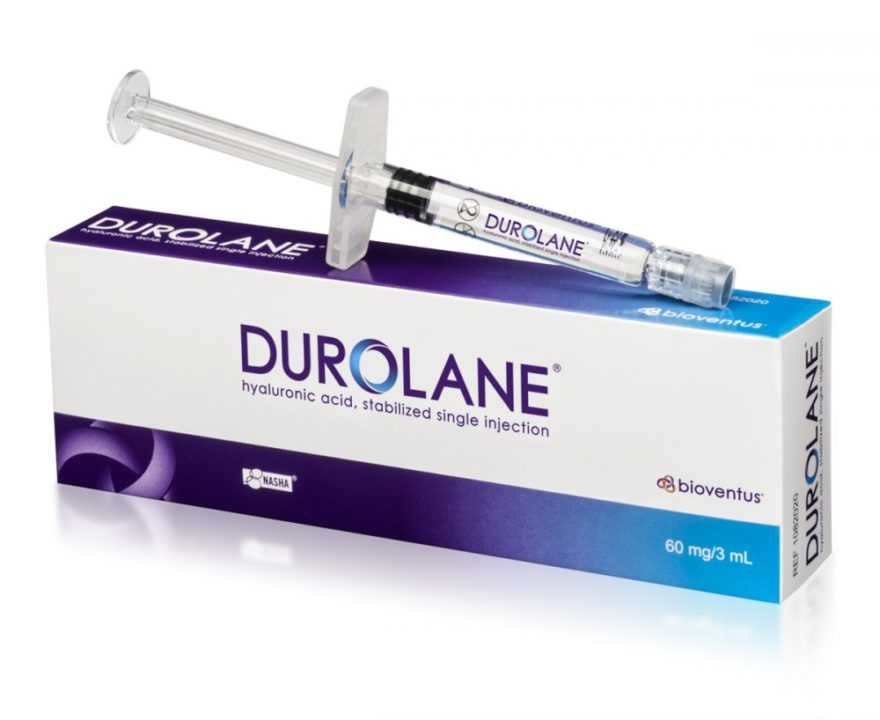
DURHAM, N.C.–(BUSINESS WIRE)–Bioventus, a global leader in orthobiologic solutions, today launched DUROLANE, its single-injection, hyaluronic acid (HA) product used for joint lubrication in the treatment of pain associated with knee osteoarthritis (OA).DUROLANE joins three-injection HA GELSYN-3™ and the five-injection HA SUPARTZ FX™ in the company’s OA portfolio making Bioventus the only orthobiologics company in the US with a single, three and five-injection HA option for treatment of knee OA.
“Introducing DUROLANE to the US market gives patients, physicians and payers access to a proven pain reliever for knee OA that has improved the lives of more than 1 million people worldwide,” said Tony Bihl, CEO of Bioventus. “In addition, DUROLANE is very safe for patients and has more level-1 clinical studies than any other single-injection HA knee therapy on the market today.”
Hyaluronic acid is a naturally occurring molecule that provides the lubrication and cushioning in a normal joint. Knee osteoarthritis involves the breakdown, or degeneration, of cartilage and the synovial fluid that cushions and lubricates joint tissues within the knee. DUROLANE has been proven to provide greater reduction in knee pain versus Synvisc-One®1* and longer lasting pain relief versus a steroid injection2. DUROLANE is also safe for repeated courses of therapy. Repeated use of DUROLANE does not increase the incidence of adverse events.2, 3
About Bioventus
Bioventus is an orthobiologics company that delivers clinically proven, cost-effective products that help people heal quickly and safely. Its mission is to make a difference by helping patients resume and enjoy active lives. Orthobiologic products from Bioventus include offerings for bone healing, bone graft and knee osteoarthritis. Its EXOGEN Ultrasound Bone Healing System uses safe, effective low intensity pulsed ultrasound (LIPUS) to stimulate the body’s natural healing process. EXOGEN has been used to treat more than 1 million patients worldwide and numerous regulatory agencies including the FDA, Health Canada, BSI, TGA, Medsafe, UAE Ministry of Health and SFDA have granted their approval of the product. Today it is the leading bone healing system in the market with complaints for lack of efficacy averaging less than 1%.
Built on a commitment to high quality standards, evidence-based medicine and strong ethical behavior, Bioventus is a trusted partner for physicians worldwide. For more information, visit www.BioventusGlobal.com and follow the company on Twitter @Bioventusglobal.
Bioventus, the Bioventus logo, EXOGEN and DUROLANE are registered trademarks and GELSYN-3, is a trademark of Bioventus LLC. SUPARTZ FX is a trademark of Seikagaku Corporation. Synvisc-One and Synvisc are registered trademarks of Genzyme Corporation.
* Some patients were treated with a three-injection Synvisc regimen. A three-injection Synvisc regimen is equivalent to one injection of Synvisc-One.
Summary of Indication for Use: DUROLANE is indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative non-pharmacological therapy or simple analgesics, e.g. acetaminophen.
Do not inject DUROLANE in patients with knee joint infections, skin diseases, or other infections in the area of the injection site. Do not administer to patients with known hypersensitivity or allergy to sodium hyaluronate preparations. Risks can include transient pain or swelling at the injection site.
DUROLANE has not been tested in pregnant or lactating women, or children. Full prescribing information can be found in product labeling, at www.DUROLANE.com, or by contacting Bioventus Customer Service at 1-800-836-4080.
References: 1. McGrath AF, McGrath AM, Jessop ZM, et al. A comparison of intra-articular hyaluronic acid competitors in the treatment of mild to moderate knee osteoarthritis. J Arthritis. 2013; 2(1):108. doi:10.4172/2167-7921.1000108. 2. Leighton R, Åkermark C, Therrien R, et. al. NASHA hyaluronic acid vs methylprednisolone for knee osteoarthritis: a prospective, multi-centre, randomized, non-inferiority trial. Osteoarthritis Cartilage. 2014; 22(1):17-25. 3. DUROLANE [package insert]. Durham, NC: Bioventus LLC; 2017.