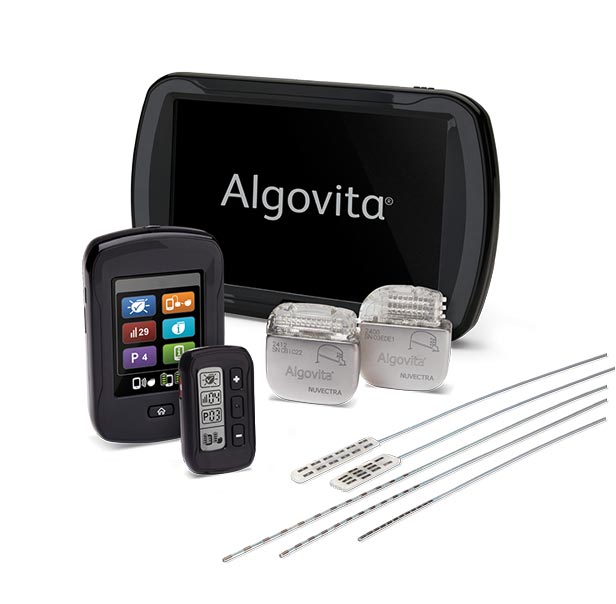
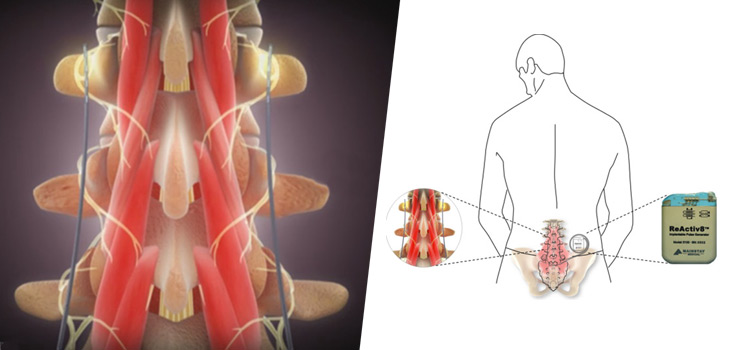
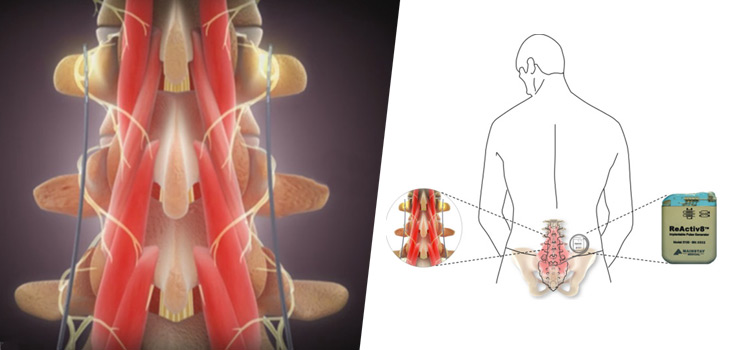
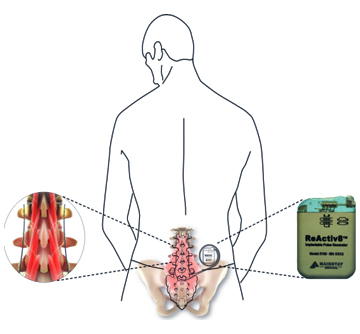
Dublin – Ireland, 5 September 2017 – Mainstay Medical International plc (“Mainstay” or the “Company”, Euronext Paris: MSTY.PA and ESM of the Irish Stock Exchange: MSTY.IE), a medical device company focused on bringing to market ReActiv8®, an implantable restorative neurostimulation system to treat disabling Chronic Low Back Pain (“CLBP”), announces that Mr. Jason Hannon will succeed Mr. Peter Crosby as Chief Executive Officer with effect from October 9, 2017. Mr. Hannon’s appointment results from the Company’s succession planning associated with the retirement of Mr. Crosby at the end of October, 2017. Mr. Hannon will also be appointed as a Director with effect from October 9, 2017.
Mr. Hannon most recently served as President and Chief Operating Officer of NuVasive (NASDAQ:NUVA), a leading medical device company focused on transforming spine surgery with minimally disruptive, procedurally-integrated solutions. His prior roles at NuVasive, Inc. include Executive Vice President of International, Executive Vice President of Business Development and Strategy, and General Counsel. During his 12 year tenure at NuVasive, the company’s commercial presence was expanded globally to more than 40 countries and revenue grew from $61M to $962M.
Dr. Oern Stuge, Chairman of Mainstay commented: “Mainstay has made tremendous progress since the founding of the Company in 2008 under Mr. Crosby’s leadership, and as we move forward to the next phase we are delighted that Jason is joining as our new CEO. Jason’s broad medical device experience has spanned areas critical to the future success of Mainstay: commercialization of new products, penetration of new markets, product innovation, strategic and financial planning, raising capital, regulatory and clinical management and the building of a high-performance culture that will attract the most talented people to our company.”
Mr. Hannon said: “Mainstay has developed a strong foundation in its scientific, clinical and regulatory accomplishments to date. The dedicated team has done the pioneering work to establish a new market – ReActiv8 seeks to help the body repair itself rather than merely masking pain. This has created the potential of bringing an entirely new option to people suffering from chronic back pain. I am impressed by the work done to get to this point, and I look forward to working with the entire team to advance the mission.”
In the period up to his retirement date, Mr. Crosby will act as Special Adviser to the new CEO, and will continue to work with the Company as a consultant up to the end of 2020. He will also continue to act as a Director until the conclusion of the Company’s Annual General Meeting to be held on Friday, 22 September 2017, when he will retire as a Director.
Mr. Crosby led Mainstay in its development of ReActiv8 from concept to commercialization. He was recruited as the Company’s first CEO in 2009 to build the Company and its team and to develop ReActiv8. Mr. Crosby led Mainstay through its Series A and Series B fundraisings to its IPO on Euronext Paris and the ESM of the Irish Stock Exchange in 2014, and its subsequent debt and equity fundraisings. In addition to fundraising, Mr. Crosby was a driving force in the development of ReActiv8 from concept stage through multiple clinical trials to CE Mark approval in 2016, start of the ReActiv8-B clinical trial to gather data for US approval, and first commercialization in Germany and Ireland in 2017. Mr. Crosby said: “I am proud of what we have achieved as a team, and I am confident that the Company will be in good hands under Jason’s leadership. I look forward to remaining involved with the Company into the future, to ensure continuity with our employees, consultants and investigators.”
Dr. Stuge concluded: “On behalf of Mainstay’s Board, management team and staff, I would like to thank Peter for his substantial contribution to the Company’s growth over the last eight years. Peter’s tireless efforts in building the Company from start-up stage through multiple fundraisings, product development and clinical trials to initial commercialization has positioned the Company well for the future. We look forward to continuing to work with Peter into the future.”
ADDITIONAL INFORMATION:
Mr Hannon holds no interest in ordinary shares of Mainstay, and, other than as set out below, there is no further information to be disclosed under schedule 2(g) and Rule 17 of the ESM Rules in respect of Mr Hannon’s appointment to the board of Mainstay.
Mr Jason Marshall Hannon (aged 45) is, or has been, a director of the following companies during the previous five years:
Previous Directorships:
Nemaris, Inc.
This announcement contains inside information within the meaning of the EU Market Abuse Regulation 596/2014
About Mainstay
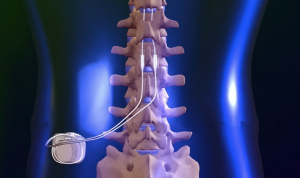
Mainstay is a medical device company focused on bringing to market an innovative implantable restorative neurostimulation system, ReActiv8®, for people with disabling Chronic Low Back Pain (CLBP). The Company is headquartered in Dublin, Ireland. It has subsidiaries operating in Ireland, the United States, Australia and Germany, and its ordinary shares are admitted to trading on Euronext Paris (MSTY.PA) and the ESM of the Irish Stock Exchange (MSTY.IE).
About the ReActiv8-B Clinical Trial
The ReActiv8-B Clinical Trial is an international, multi-center, prospective randomized sham controlled blinded trial with one-way crossover conducted under an Investigational Device Exemption (IDE). The ReActiv8-B Clinical Trial is designed to generate data to form part of the Pre-Market Approval Application (PMAA) of ReActiv8 to the FDA. Further details can be found at https://clinicaltrials.gov/show/NCT02577354
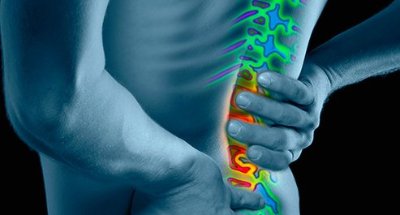
About Chronic Low Back Pain
One of the recognized root causes of CLBP is impaired control by the nervous system of the muscles that dynamically stabilize the spine in the low back, and an unstable spine can lead to back pain. ReActiv8 is designed to electrically stimulate the nerves responsible for contracting these muscles and thereby help to restore muscle control and improve dynamic spine stability, allowing the body to recover from CLBP.
People with CLBP usually have a greatly reduced quality of life and score significantly higher on scales for pain, disability, depression, anxiety and sleep disorders. Their pain and disability can persist despite the best available medical treatments, and only a small percentage of cases result from an identified pathological condition or anatomical defect that may be correctable with spine surgery. Their ability to work or be productive is seriously affected by the condition and the resulting days lost from work, disability benefits and health resource utilization put a significant burden on individuals, families, communities, industry and governments.
Further information can be found at www.mainstay‑medical.com
CAUTION – in the United States, ReActiv8 is limited by federal law to investigational use only.
PR and IR Enquiries:
Consilium Strategic Communications (international strategic communications – business and trade media)
Chris Gardner, Mary-Jane Elliott, Jessica Hodgson, Hendrik Thys
Tel: +44 203 709 5700 / +44 7921 697 654
Email: mainstaymedical@consilium-comms.com
FTI Consulting (for Ireland)
Jonathan Neilan
Tel: +353 1 765 0886
Email: jonathan.neilan@fticonsulting.com
NewCap (for France)
Louis-Victor Delouvrier
Tel: +: +33 1 44 71 98 53
Email: lvdelouvrier@newcap.fr
AndreasBohne.Com/Kötting Consulting (for Germany)
Andreas Bohne
Tel : +49 2102 1485368
Email : abo@andreasbohne.com
Investor Relations:
LifeSci Advisors, LLC
Brian Ritchie
Tel: +1 (212) 915-2578
Email: britchie@lifesciadvisors.com
ESM Advisers:
Davy
Fergal Meegan or Barry Murphy
Tel: +353 1 679 6363
Email: fergal.meegan@davy.ie or barry.murphy2@davy.ie
Forward looking statements
This announcement includes statements that are, or may be deemed to be, forward looking statements. These forward looking statements can be identified by the use of forward looking terminology, including the terms “anticipates”, “believes”, “estimates”, “expects”, “intends”, “may”, “plans”, “projects”, “should”, “will”, or “explore” or, in each case, their negative or other variations or comparable terminology, or by discussions of strategy, plans, objectives, goals, future events or intentions. These forward looking statements include all matters that are not historical facts. They appear throughout this announcement and include, but are not limited to, statements regarding the Company’s intentions, beliefs or current expectations concerning, among other things, the Company’s results of operations, financial position, prospects, financing strategies, expectations for product design and development, regulatory applications and approvals, reimbursement arrangements, costs of sales and market penetration.
By their nature, forward looking statements involve risk and uncertainty because they relate to future events and circumstances. Forward looking statements are not guarantees of future performance and the actual results of the Company’s operations, and the development of its main product, the markets and the industry in which the Company operates, may differ materially from those described in, or suggested by, the forward looking statements contained in this announcement. In addition, even if the Company’s results of operations, financial position and growth, and the development of its main product and the markets and the industry in which the Company operates, are consistent with the forward looking statements contained in this announcement, those results or developments may not be indicative of results or developments in subsequent periods. A number of factors could cause results and developments of the Company to differ materially from those expressed or implied by the forward looking statements including, without limitation, the successful launch and commercialization of ReActiv8®, the progress and success of the ReActiv8-B Clinical Trial, general economic and business conditions, the global medical device market conditions, industry trends, competition, changes in law or regulation, changes in taxation regimes, the availability and cost of capital, the time required to commence and complete clinical trials, the time and process required to obtain regulatory approvals, currency fluctuations, changes in its business strategy, political and economic uncertainty. The forward-looking statements herein speak only at the date of this announcement.